How to Find the Expiration on Test Reagents: Easy Steps to Locate and Read Expiry Dates
When I open a box of test reagents I always check the expiration date before getting started. It’s easy to overlook but using expired reagents can lead to unreliable results and wasted time. That’s why knowing exactly where to find the expiration date is so important.
I’ve noticed that manufacturers don’t always put the date in the same spot or use the same format. Sometimes it’s printed on the label other times it’s stamped on the box or even hidden in a code. If you’re not sure what to look for you might miss it. Let me share some simple tips to help you quickly spot the expiration date on your test reagents so you can work with confidence every time.
Importance of Checking Test Reagent Expiration

Verifying test reagent expirations protects test accuracy and prevents flawed results. Expired reagents lose effectiveness, causing inconsistent or false data during testing. Many organizations like the CDC and FDA link reagent reliability with valid expiration dates, referencing standards for laboratory quality control.
Incorrect results from expired reagents risk misdiagnosis, regulatory violations, and financial loss. In regulated settings—examples include medical labs and food safety testing—using expired materials can trigger compliance penalties.
Routine expiration checks cut waste by identifying products that can’t be used. Monitoring ensures inventory rotates properly, which reduces disposal costs and supports safety practices.
| Reason for Checking | Impact Example | Source |
|---|---|---|
| Accuracy assurance | Reliable glucose or pH test results | CDC, WHO |
| Regulatory compliance | Laboratory inspection standards | FDA, CLIA |
| Cost control | Fewer discarded expired reagents | CAP, APHL |
Manufacturers disclose expiration dates on test reagent labels, outer packaging, or product inserts. Dates appear as MM/YYYY, DD/MM/YY, or coded formats. Misinterpreting these can affect quality management efforts. Regular training on date verification maintains lab integrity and confidence in analytical processes.
Common Locations of Expiration Dates on Test Reagents
Expiration dates on test reagents often appear in different places and formats. I use these main locations to find expiration data quickly and maintain lab quality.
Packaging and Bottles
Labeling on test reagent containers usually displays expiration information. I often see dates printed on:
- Primary bottles or vials
- Outer boxes or plastic shrink wraps
- Bottle caps or bottle bases
Location and visibility can vary by manufacturer and batch. Expiration formats may include month/year (e.g., 05/2026), day/month/year (e.g., 12/05/2026), or a sequence number. Industry standards like ISO 15223-1 recommend standard date placement but compliance isn’t universal.
Typical Expiration Date Formats by Container Type
| Container Type | Common Location | Example Format |
|---|---|---|
| Glass/Plastic Vial | Label front, cap | 2026-05, 05/2026 |
| Outer Box | Near barcode, corner | YYMMDD, MM/YYYY |
| Sachet/Pouch | Edge, fold, back | YYYY.MM.DD |
Product Inserts and Documentation
Product inserts and official documents often include critical expiration details. I find that manufacturers provide this in:
- Instructions for use or quick-start guides
- Certificates of analysis or conformity
- Safety data sheets (SDS)
Inserts can confirm or clarify the printed date. Sometimes, inserts specify the time frame for use after opening, in addition to sealed expiration.
Digital Resources and Manufacturer Websites
Digital resources help locate or verify expiration dates for test reagents when labels are missing or unclear. I access:
- Manufacturer product pages and digital catalogs
- Online technical support databases
- Barcode or lot tracking systems (entered on company websites)
Some brands offer QR codes or batch lookup tools to confirm reagent validity. In regulated industries, I rely on these tools for traceability and compliance.
| Digital Resource | Description |
|---|---|
| QR code/Barcode lookup | Scan code to access date, lot, or recall info |
| Manufacturer support/FAQ | Online manuals, expiry lists by product |
| Regulatory portals (FDA, CDC) | Recall alerts and shelf-life updates |
Steps to Identify Expiration Dates
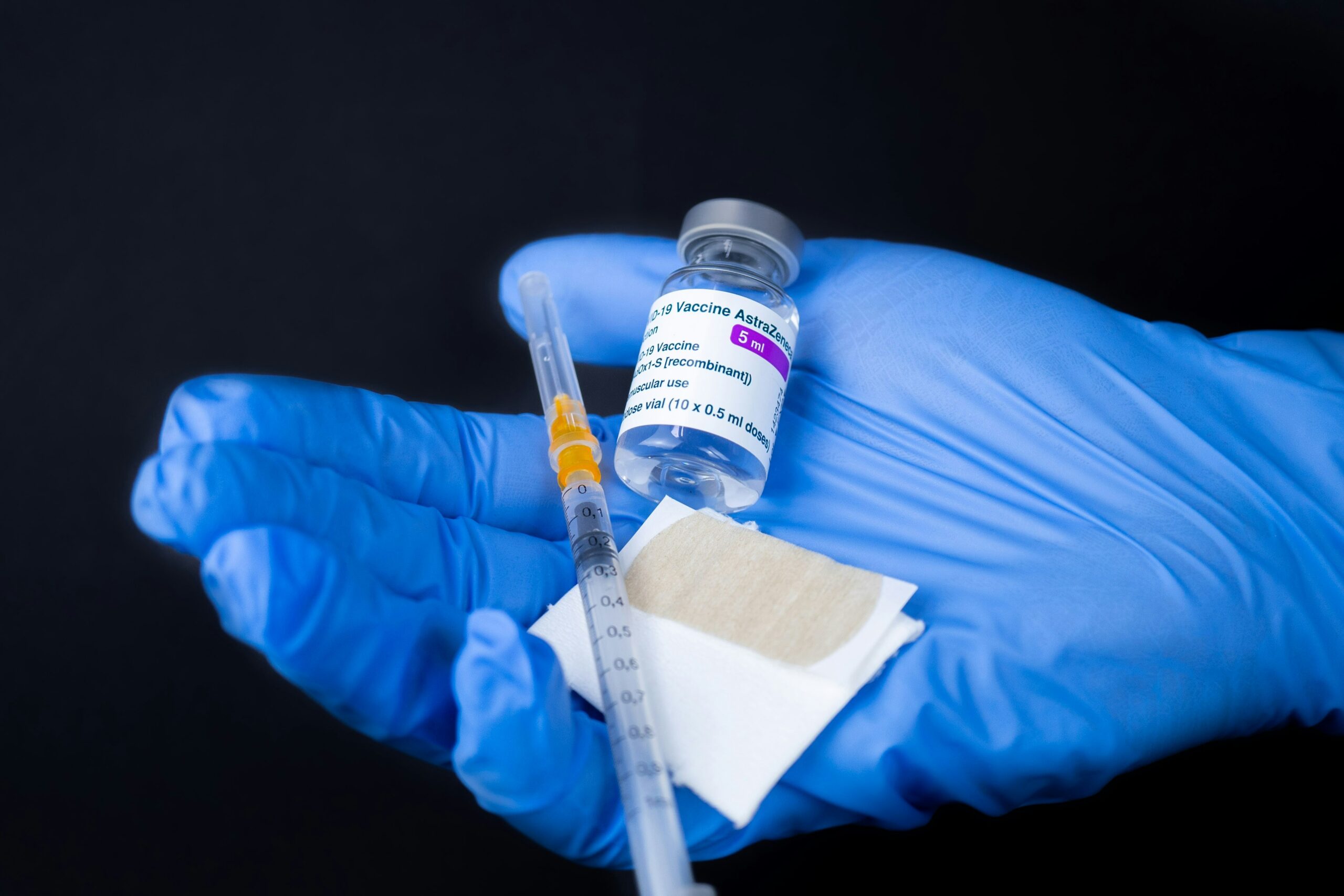
Locating and interpreting expiration dates on test reagents supports reliable results and regulatory compliance. I check the following aspects for accuracy and traceability.
Deciphering Date Formats
Manufacturers print expiration dates using several formats, which can cause confusion. I reference this table to distinguish between common date formats on reagent labels:
| Printed Format | Example | Representation | Interpretation |
|---|---|---|---|
| MM/DD/YYYY | 06/01/2024 | Month/Day/Year | Expires June 1, 2024 |
| DD/MM/YYYY | 01/06/2024 | Day/Month/Year | Expires June 1, 2024 |
| YYYY-MM | 2024-06 | Year-Month | Expires June 30, 2024 |
| MMM YYYY | JUN 2024 | Month (Abbrev.) Year | Expires June 30, 2024 |
| YYMMDD | 240601 | Year(2)-Month-Day | Expires June 1, 2024 |
| YYYYMMDD | 20240601 | Year-Month-Day | Expires June 1, 2024 |
Manufacturers often indicate the format next to the printed date or in the instructions. I confirm the convention if multiple formats are present.
Understanding Lot Numbers Versus Expiry Dates
Test reagent labeling usually displays both a lot number and an expiration date. I distinguish between the two by these indicators:
- Expiry dates always follow a recognizable calendar format, examples include “Exp,” “Use By,” or a specific date sequence.
- Lot numbers contain letters, numbers, or symbols unique to that production batch, examples include “Lot,” “#,” or “Batch.”
I avoid confusing these values by cross-referencing them with the product documentation. Only expiration dates indicate when reagent use is no longer valid. If I ever see ambiguous codes, manufacturer support clarifies which is the true expiration reference.
What to Do If Expiration Information Is Missing
Contacting the manufacturer provides the fastest way to get official expiration details if expiration information isn’t visible on your test reagent or packaging. I use the product name, lot number, and purchase date when reaching out to technical support or customer service teams. Most major reagent suppliers like Thermo Fisher Scientific, Sigma-Aldrich, and Bio-Rad maintain helplines and online inquiry forms for this purpose.
Reviewing product inserts or electronic documentation can uncover hidden or overlooked expiration guidance. I search for digital PDFs of instructions on the manufacturer’s website by entering the product catalog or part number. Downloaded technical sheets often list shelf life, recommended storage conditions, and date-of-opening guidelines.
Consulting purchase records helps validate stability claims if vendor documentation is unavailable. I refer to the original invoice, packing slip, or procurement database entry for receipt dates and typical shelf life. Internal records sometimes state a recommended period after opening or delivery.
Isolating reagents with unidentified expiration dates prevents erroneous results. I label affected products as “expiration unknown” and move them to a designated review area until I determine their status.
Following regulatory guidance from authorities like the FDA or CDC ensures compliance for reagents lacking expiration data in clinical or food safety labs. I refer to organization-specific policies for managing untraceable products, which may include retesting with new reagents, documentation, or disposal.
Below is a table summarizing the steps to take when expiration information is missing:
| Step | Action | Example/Detail |
|---|---|---|
| Contact manufacturer | Provide lot number, product name, and purchase date | Email technical support at Sigma-Aldrich |
| Review official documentation | Search for product inserts or download digital instructions | Find PDF on Thermo Fisher website |
| Check purchase records | Look up procurement history for shelf life information | Review invoice or ERP log |
| Isolate unverified reagents | Separate and label as “expiration unknown” | Move to a designated lab bin |
| Follow regulatory protocols | Adhere to CDC/FDA or internal lab guidelines | Document and arrange for disposal or retesting |
Tips for Managing Reagent Expiration in the Lab
Proper tracking and storage optimize reagent expiration management in the lab. I use these structured practices to reduce risk and keep lab workflows efficient:
- Implement tracking systems with digital logs or inventory management software like Quartzy to automate expiration reminders and trace lot numbers.
- Store reagents by expiry date, placing soon-to-expire items at the front to ensure first-in, first-out (FIFO) usage and minimize waste.
- Perform regular audits, checking expiration dates on primary bottles, caps, boxes, and inserts each month to catch outdated reagents quickly.
- Train staff annually on locating and interpreting expiration formats, changes in labeling, and proper documentation for consistent protocol adherence.
- Label opened containers with the date of opening if required, following manufacturer guidance for usage-after-opening intervals.
These steps help maintain reliable analytical results, regulatory compliance, and proper reagent inventory turnover.
Example Lab Reagent Expiration Management Schedule
| Task | Frequency | Responsible Person | Method |
|---|---|---|---|
| Inventory Review | Monthly | Lab Manager | Manual check of all stocked reagents |
| Digital Expiry Tracking | Ongoing | Designated Staff | Quartzy inventory reminders |
| FIFO Restocking | Upon Arrival | Stockroom Staff | Physical placement by expiration |
| Staff Expiration Training | Annually | QA Lead | Hands-on walk-through |
Common Expiry Labeling Mistakes in Labs
| Mistake | Result | Solution |
|---|---|---|
| Ignoring product inserts | Missed critical date info | Always review inserts first |
| Mixing lot and expiry numbers | Misinterpreted reagent status | Use decoding tables |
| Forgetting opened date marking | Inaccurate shelf life tracking | Use visible onset-date labels |
| Not isolating unlabeled reagents | Accidental use of expired stock | Segregate as “exp unknown” |
Regularly using these tables and documented practices ensures I keep expiration-based errors out of critical test workflows.
Conclusion
Staying vigilant about test reagent expiration dates is one of the simplest ways I protect the quality of my results and maintain compliance. By making expiration checks part of my routine and using every available resource to verify dates I keep my lab running smoothly and avoid costly mistakes.
I’ve found that investing a little time in training and organizing my inventory pays off in both confidence and accuracy. With these habits in place I can trust my results and focus on what matters most—delivering reliable data every time.
Frequently Asked Questions
Why is checking the expiration date on test reagents important?
Checking expiration dates ensures test results are accurate and reliable. Using expired reagents can cause inconsistent or false data, potentially leading to misdiagnosis, regulatory issues, or financial losses.
Where can I find the expiration date on test reagents?
Expiration dates are usually printed on the main bottle, outer packaging, bottle cap, or product insert. Some manufacturers also provide expiration details via QR codes, online databases, or their website.
How do manufacturers format expiration dates?
Expiration dates can appear as YYYY-MM-DD, MM-YYYY, or MM/DD/YYYY, depending on the manufacturer. They are distinct from lot numbers, which indicate the batch but not the expiration.
What if I can’t find the expiration date on my reagent?
If expiration info is missing, isolate the reagent and label it as “expiration unknown.” Check product inserts, digital resources, or contact the manufacturer for details. Always follow regulatory guidance.
What’s the difference between an expiration date and a lot number?
An expiration date indicates until when the reagent is guaranteed to work reliably, usually in a date format. A lot number identifies the production batch and does not reflect product shelf life.
Are digital resources reliable for checking reagent expirations?
Yes, digital resources like manufacturer websites, QR codes, and support databases are reliable ways to verify expiration dates—especially if physical labels are damaged or unclear.
What are best practices for managing reagent expiration in the lab?
Use digital tracking systems, regularly audit your inventory, organize reagents by expiration dates, label opened containers, and train staff annually on handling and checking expirations.
What should I do if I accidentally use an expired reagent?
Document the incident, isolate affected results, retest with valid reagents, and inform relevant stakeholders. Follow your lab’s protocol and regulatory guidance for corrective actions.
How can I prevent mistakes with expiration labeling?
Implement consistent labeling, double-check dates during audits, train staff, and separate near-expiry or expired reagents from active stock. Use inventory management tools for added accuracy.
Why do regulatory agencies like the FDA and CDC stress reagent expiration checks?
Regulators require valid expiration checks to ensure test reliability, patient safety, and proper data integrity in labs, especially in medical and food safety industries. Compliance helps avoid violations and maintains quality standards.
